Table of Characteristics Functional Imaging Techniques
Electrical Based Techniques
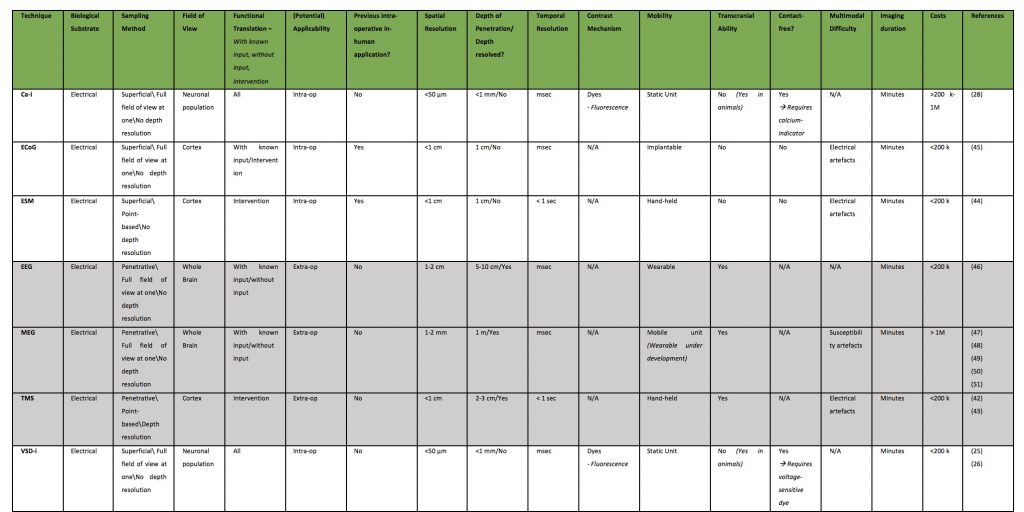
Blood Dynamics Based Techniques
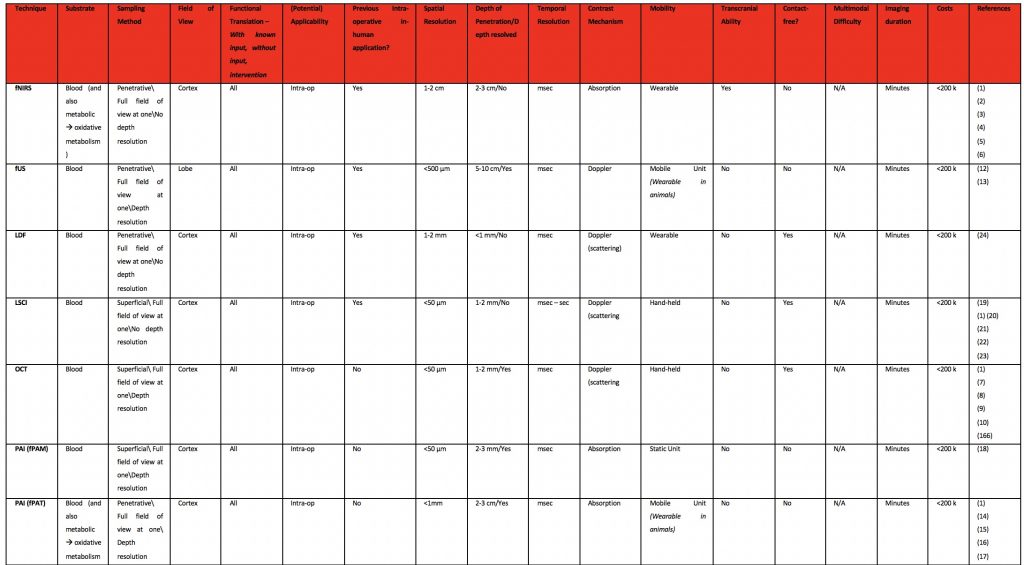
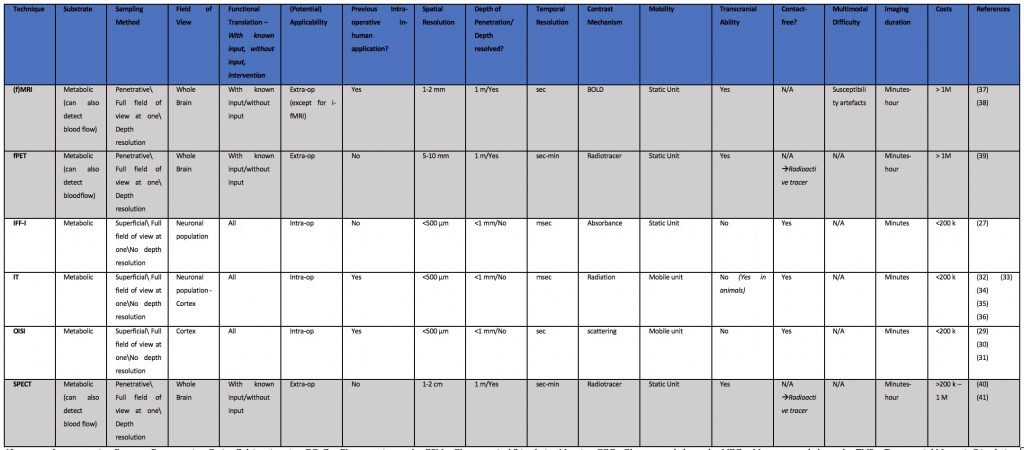
Metabolism Based Techniques
References
- Devor A, Sakadžić S, Srinivasan VJ, Yaseen MA, Nizar K, Saisan PA, et al. Frontiers in optical imaging of cerebral blood flow and metabolism. Journal of Cerebral Blood Flow and Metabolism. 2012.
- Jobsis F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science (80- ). 1977;
- Franceschini MA, Boas DA. Noninvasive measurement of neuronal activity with near-infrared optical imaging. Neuroimage. 2004;
- Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;
- Qiu T, Hameed NUF, Peng Y, Wang S, Wu J, Zhou L. Functional near-infrared spectroscopy for intraoperative brain mapping. Neurophotonics. 2019;
- Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Vol. 63, NeuroImage. 2012. p. 921–35.
- Aumann S, Donner S, Fischer J, Müller F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In: High Resolution Imaging in Microscopy and Ophthalmology. 2019.
- Carrasco-Zevallos OM, Viehland C, Keller B, Draelos M, Kuo AN, Toth CA, et al. Review of intraoperative optical coherence tomography: technology and applications [Invited]. Biomed Opt Express. 2017;
- Ramakonar H, Quirk BC, Kirk RW, Li J, Jacques A, Lind CRP, et al. Intraoperative detection of blood vessels with an imaging needle during neurosurgery in humans. Sci Adv. 2018 Dec 20;4(12):eaav4992.
- Men J, Huang Y, Solanki J, Zeng X, Alex A, Jerwick J, et al. Optical Coherence Tomography for Brain Imaging and Developmental Biology. IEEE J Sel Top Quantum Electron. 2016;
- Chen Y, Aguirre AD, Ruvinskaya L, Devor A, Boas DA, Fujimoto JG. Optical coherence tomography (OCT) reveals depth-resolved dynamics during functional brain activation. J Neurosci Methods. 2009;
- Soloukey S, Vincent AJPE, Satoer DD, Mastik F, Smits M, Dirven CMF, et al. Functional Ultrasound (fUS) During Awake Brain Surgery: The Clinical Potential of Intra-Operative Functional and Vascular Brain Mapping. Front Neurosci. 2020;
- Imbault M, Serroune H, Gennisson JL, Tanter M, Chauvet D, Capelle L, et al. Functional Ultrasound Imaging of the Human Brain Activity: An Intraoperative Pilot Study for Cortical Functional Mapping. In: 2016 Ieee International Ultrasonics Symposium. New York: Ieee; 2016. (IEEE International Ultrasonics Symposium).
- Zhang P, Li L, Lin L, Hu P, Shi J, He Y, et al. High-resolution deep functional imaging of the whole mouse brain by photoacoustic computed tomography in vivo. J Biophotonics. 2018;
- Hariri A, Tavakoli E, Adabi S, Gelovani J, Avanaki MRN. Functional photoacoustic tomography for neonatal brain imaging: developments and challenges. In: Photons Plus Ultrasound: Imaging and Sensing 2017. 2017.
- Tang J, Coleman JE, Dai X, Jiang H. Wearable 3-D Photoacoustic Tomography for Functional Brain Imaging in Behaving Rats. Sci Rep. 2016;114:359–364.
- Yao J, Wang L V. Photoacoustic tomography: Fundamentals, advances and prospects. Contrast Media and Molecular Imaging. 2011.
- Liao L De, Li ML, Lai HY, Shih YYI, Lo YC, Tsang S, et al. Imaging brain hemodynamic changes during rat forepaw electrical stimulation using functional photoacoustic microscopy. Neuroimage. 2010;
- Briers D, Duncan DD, Hirst E, Kirkpatrick SJ, Larsson M, Steenbergen W, et al. Laser speckle contrast imaging: theoretical and practical limitations. J Biomed Opt. 2013;
- Klijn E, Hulscher HC, Balvers RK, Holland WP, Bakker J, Vincent AJ, et al. Laser speckle imaging identification of increases in cortical microcirculatory blood flow induced by motor activity during awake craniotomy. J Neurosurg. 2013;118(2):280–6.
- Dunn AK. Laser speckle contrast imaging of cerebral blood flow. Ann Biomed Eng. 2012;
- Senarathna J, Rege A, Li N, Thakor N V. Laser speckle contrast imaging: Theory, instrumentation and applications. IEEE Rev Biomed Eng. 2013;
- Heeman W, Steenbergen W, van Dam GM, Boerma EC. Clinical applications of laser speckle contrast imaging: a review. J Biomed Opt. 2019;
- Raabe A, Van De Ville D, Leutenegger M, Szelényi A, Hattingen E, Gerlach R, et al. Laser Doppler imaging for intraoperative human brain mapping. Neuroimage. 2009;44(4):1284–9.
- Ferezou I, Matyas F, Petersen CCH. Imaging the Brain in Action: Real-Time Voltage- Sensitive Dye Imaging of Sensorimotor Cortex of Awake Behaving Mice. In Vivo Optical Imaging of Brain Function. 2009.
- Kang J, Zhang HK, Kadam SD, Fedorko J, Valentine H, Malla AP, et al. Transcranial Recording of Electrophysiological Neural Activity in the Rodent Brain in vivo Using Functional Photoacoustic Imaging of Near-Infrared Voltage-Sensitive Dye. Front Neurosci. 2019;
- Husson TR, Issa NP. Functional Imaging with Mitochondrial Flavoprotein Autofluorescence: Theory, Practice, and Applications. In Vivo Optical Imaging of Brain Function. 2009.
- Chen Q, Cichon J, Wang W, Qiu L, Lee SJR, Campbell NR, et al. Imaging Neural Activity Using Thy1-GCaMP Transgenic Mice. Neuron. 2012;
- Sato K, Nariai T, Momose-Sato Y, Kamino K. Intraoperative intrinsic optical imaging of human somatosensory cortex during neurosurgical operations. Neurophotonics. 2017;4(3).
- Lu HD, Chen G, Cai J, Roe AW. Intrinsic signal optical imaging of visual brain activity: Tracking of fast cortical dynamics. Neuroimage. 2017;
- Zepeda A, Arias C, Sengpiel F. Optical imaging of intrinsic signals: Recent developments in the methodology and its applications. Journal of Neuroscience Methods. 2004.
- Rojas EDFR, Ochoa EM, López RL, Díaz LL. Infrared thermography brain mapping surveillance in vascular neurosurgery for anterior communicating artery aneurysm clipping. Surg Neurol Int. 2018;
- Hoffmann N, Radev Y, Koch E, Petersohn U, Steiner G, Kirsch M. Intraoperative mapping of the sensory cortex by time-resolved thermal imaging. Biomed Tech. 2018;
- Shevelev IA. Functional imaging of the brain by infrared radiation (thermoencephaloscopy). Progress in Neurobiology. 1998.
- Gorbach AM, Heiss J, Kufta C, Sato S, Fedio P, Kammerer WA, et al. Intraoperative infrared functional imaging of human brain. ANN NEUROL. 2003;54(3):297–309.
- Shevelev IA, Tsicalov EN, Gorbach AM, Budko KP, Sharaev GA. Thermoimaging of the brain. J Neurosci Methods. 1993;
- Rutten GJ, Ramsey NF. The role of functional magnetic resonance imaging in brain surgery. Neurosurg Focus. 2010;
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008.
- Ballanger B, van Eimeren T, Strafella AP. Diagnostic PET in Image Guided Neurosurgery. In: Textbook of Stereotactic and Functional Neurosurgery. 2009.
- Lammertsma AA. PET/SPECT: Functional imaging beyond flow. In: Vision Research. 2001.
- Devous MD. SPECT Functional Brain Imaging: Technical Considerations. J Neuroimaging. 1995;
- Sliwinska MW, Vitello S, Devlin JT. Transcranial magnetic stimulation for investigating causal brain-behavioral relationships and their time course. J Vis Exp. 2014;
- Krieg SM. Navigated transcranial magnetic stimulation in neurosurgery. Navigated Transcranial Magnetic Stimulation in Neurosurgery. 2017.
- Pouratian N, Cannestra AF, Bookheimer SY, Martin NA, Toga AW. Variability of intraoperative electrocortical stimulation mapping parameters across and within individuals. J NEUROSURG. 2004;101(3):458–66.
- Korostenskaja M, Chen PC, Salinas CM, Westerveld M, Brunner P, Schalk G, et al. Real-time functional mapping: Potential tool for improving language outcome in pediatric epilepsy surgery – Case report. J Neurosurg Pediatr. 2014;14(3):287–95.
- Ferree TC, Clay MT, Tucker DM. The spatial resolution of scalp EEG. Neurocomputing. 2001;
- Boto E, Hill RM, Rea M, Holmes N, Seedat ZA, Leggett J, et al. Measuring functional connectivity with wearable MEG. bioRxiv. 2020;
- Sommer B, Roessler K, Rampp S, Hamer HM, Bluemcke I, Buchfelder M, et al. Magnetoencephalography-Guided frontal lobe epilepsy surgery using neuronavigation and intraoperative MR imaging. Epilepsia. 2016;57:25.
- Ellis DG, White ML, Hayasaka S, Warren DE, Wilson TW, Aizenberg MR. Accuracy analysis of fMRI and MEG activations determined by intraoperative mapping. Neurosurg Focus. 2020;
- Mäkelä JP, Forss N, Jääskeläinen J, Kirveskari E, Korvenoja A, Paetau R. Magnetoencephalography in neurosurgery. Neurosurgery. 2006.
- Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS, et al. Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation Clinical article. J Neurosurg. 2012;117(2):354–62.
