MEG
Moderated by Jyrki Mäkelä, Senior Researcher at BioMag Laboratory HUS Medical Imaging Center, Helsinki University Hospital, Finland
Magnetoencephalography (MEG) is a non-invasive technique which exploits the fact that the electrical activity produced by the brain creates minuscule but detectable magnetic fields (1,2).
Technical Parameters
The MEG-signal which is detectable extracranially, is produced by intra-cellular current flow in the active neurons (1). Given the relatively small magnetic fields that are generated by brain activity, the detectors used to pick up this signal need to be very sensitive (1). In fact, the MEG-magnetometers are made superconductive by liquid helium and data are often collected in shielded rooms to prevent noise and ensure signal quality.
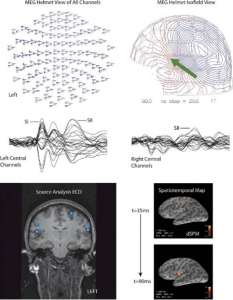
The temporal resolution of MEG is excellent (milliseconds), even allowing to follow-up brain activation sequences. The spatial resolution is more limited (several millimeters) given the challenge of the ‘inverse problem’: determining the sources of the magnetic field when measured extracranially (2). Multi-channel MEG is, however, powerful enough to facilitate recordings spanning the full cortical surface.
Biological Substrate
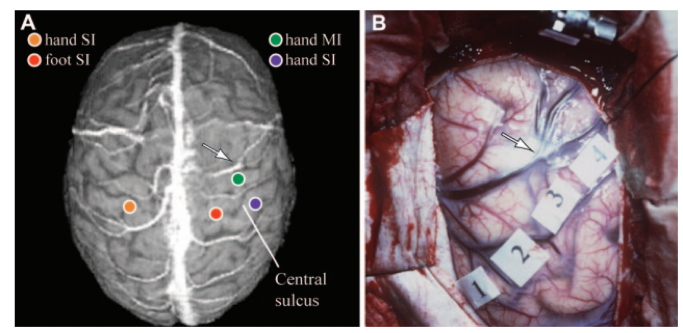
The signal captured in MEG is generated mainly by dipolar currents associated with dendritic excitatory and inhibitory postsynaptic potentials of mostly pyramidal cells of the cerebral cortex (1,3). As such, MEG is a direct measurement of brain activity. Unlike Electroencephalography (EEG), which will be discussed in the following chapters, MEG preferentially detects activity in superficial, non-radial areas of cortex, such as the fissural cortex of the cerebral hemispheres. This is particularly advantageous if the area of interest is also radial, such as the primary somatosensory cortex (3).
Intra-operative applicability
So far, MEG has been used for multiple clinical purposes including pre-operative detection of epileptic zones (1) or mapping of e.g. sensory, motor and language-related functions in preparation for awake surgery (4–11). For this purpose, the MEG-device coordinate system is registered to the anatomical coordinate system of the subject’s head or the structural MRI. This registration comes with challenges in terms of spatial accuracy, but these seem manageable and similar to the challenges for other functional modalities such as fMRI (1). The obtained preoperative functional landmarks and localizations of epileptiform interictal and ictal activity (12) can easily be incorporated into MRIs in neuronavigation systems for intraoperative use (13).
The main bottlenecks for its wide-spread intra-operative application seem to be a combination of infrastructure-related factors. Firstly, the costs of the MEG-unit, which together with the additional adjustment such as a shielded room is estimated to cost several million dollars (1). It is worth noting that this cost does not exceed that of a high-field MRI unit. Additionally, the MEG-unit is sizeable, static and easily susceptible to noise and artifacts (1), making actual intra-operative recordings without significant surgical workflow disruption difficult. Literature does describe the development of mobile, wearable alternatives of MEG, which might improve the intra-operative potential in the future (14,15).
References
- Mäkelä JP, Forss N, Jääskeläinen J, Kirveskari E, Korvenoja A, Paetau R. Magnetoencephalography in neurosurgery. Neurosurgery. 2006.
- Mäkelä JP. Bioelectric Measurements: Magnetoencephalography. In: Comprehensive Biomedical Physics. Vol 5. ; 2014.
- Hoyos A de, Portillo J, Marín P, Á. García M, Maestú F, Lafuente J, et al. Improving the spatial resolution of magnetoencephalography images. Neurol Neurosurg. 2018;
- Stufflebeam SM. Clinical magnetoencephalography for neurosurgery. Neurosurgery Clinics of North America. 2011.
- Ganslandt O, Fahlbusch R, Nimsky C, Kober H, Möller M, Steinmeier R, et al. Functional neuronavigation with magnetoencephalography: Outcome in 50 patients with lesions around the motor cortex. J Neurosurg.1999;91(1):73–9.
- Korvenoja A, Kirveskari E, Aronen HJ, Avikainen S, Brander A, Huttunen J, et al. Sensorimotor cortex localization: Comparison of magnetoencephalography, functional MR imaging, and intraoperative cortical mapping. 2006;241(1):213–22.
- Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS, et al. Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation Clinical article. J Neurosurg. 2012;117(2):354–62.
- Schiffbauer H, Berger MS, Ferrari P, Freudenstein D, Rowley HA, Roberts TPL. Preoperative magnetic source imaging for brain tumor surgery: A quantitative comparison with intraoperative sensory and motor mapping. J NEUROSURG. 2002;97(6):1333–42.
- Nagarajan S, Kirsch H, Lin P, Findlay A, Honma S, Berger MS. Preoperative localization of hand motor cortex by adaptive spatial filtering of magnetoencephalography data. J Neurosurg. 2008;
- Ottenhausen M, Krieg SM, Meyer B, Ringel F. Functional preoperative and intraoperative mapping and monitoring: increasing safety and efficacy in glioma surgery. Neurosurg Focus. 2015;38(1):E3.
- Kreidenhuber R, De Tiège X, Rampp S. Presurgical functional cortical mapping using electromagnetic source imaging. Front Neurol. 2019;
- Medvedovsky M, Taulu S, Gaily E, et al. Sensitivity and specificity of seizure-onset zone estimation by ictal magnetoencephalography. Epilepsia. 2012;53(9).
- Vitikainen AM, Lioumis P, Paetau R, et al. Combined use of non-invasive techniques for improved functional localization for a selected group of epilepsy surgery candidates. Neuroimage. 2009;45(2).
- Boto E, Hill RM, Rea M, Holmes N, Seedat ZA, Leggett J, et al. Measuring functional connectivity with wearable MEG. bioRxiv. 2020;
- Boto E, Holmes N, Leggett J, et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature. 2018;555(7698).
