fMRI
Moderated by Esther Warnert, Assistant Professor and Principal Investigator of Bench-to-Bedside MRI, Erasmus MC, Rotterdam, The Netherlands
Functional Magnetic Resonance Imaging (fMRI) is the most widespread, non-invasive functional imaging technique used as a pre-operative brain-mapping tool to guide neurosurgical treatment decisions (1). fMRI measures the Blood Oxygenation Level Dependent (BOLD)– effect as a consequence of neuronal activation-induced changes in Cerebral Blood Flow and Volume (CBF and CBV) (2).
Technical Parameters
MRI depends on powerful magnets to produce a magnetic field strong enough to force protons in the body to align to it. Then, a radiofrequency field is introduced, which forces the protons to spin out of their equilibrium, straining against the pull off the magnetic field. When the radiofrequency field is turned off, the protons realign to the field again, releasing electromagnetic energy on their way. The speed of this energy release is detected by the MRI machine and used to distinguish between tissue types.
In fMRI, we exploit the fact that oxygenated hemoglobin is diamagnetic, i.e. does not affect the local magnetic field, whereas deoxygenated hemoglobin is paramagnetic, i.e. affects the local magnetic field. This difference in magnetic properties allows us to discriminate between blood signal depending on its degree of oxygenation with fMRI, which in turn is an indirect measure of the level of neuronal activity through the process of neurovascular coupling (NVC) (Figure 1).
Important to note is that the BOLD-signal is a signal with significant latency. As you can read in the chapter on Optical Intrinsic Signal Imaging (OISI), the local activation of neurons is initially met with a decrease of blood oxygenation, known as the ‘initial dip’. The bulk of the BOLD-signal, however, consist of the 1-2 sec delayed hemodynamic response, where through increase in blood flow and surrounding arteriole dilatation, ‘fresh’ oxygenated blood is provided, which peaks several seconds after the original activation (2–4).
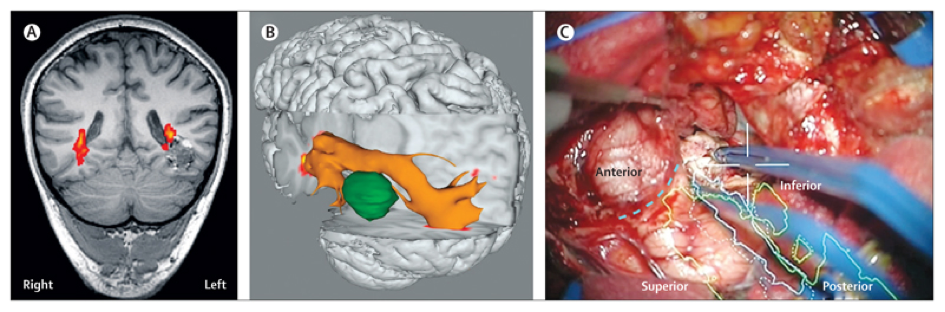
fMRI as a technique in the clinical setting has a spatial resolution in the millimeter range and a temporal resolution of 1-2 seconds. However, based on biological grounds we might consider the spatial resolution to be even larger than that, as will be discussed below. Acquisition time for a single functional task usually consists of task patterns of several minutes, with total time spent in the scanner for combined structural and functional MRIs in preparation for surgery being close to an hour. Often, pre-operative fMRI mapping for neurosurgical procedures also includes structural Diffusion Tensor Imaging (DTI, also known as tractography), where the anisotropic nature of water diffusion in fibrous tissues is exploited to visualize white matter tracts which are part of functional networks (5) (Figure 2). As such, additional functionally valuable, deeper brain structures such as the arcuate fasciculus or the pyramidal tract can be morphologically visualized and intra-operatively co-registered to the patient.
Biological Substrate
fMRI relies on the BOLD-effect, a metabolism-based signal which serves as an indirect measure of neuronal activity. Often, specific tasks in specific task patterns are used to evoke activity in functional regions of interest.
In physiological situation, a local increase in neuronal activity is accompanied by a metabolism-driven need for oxygen. As such, initially, a local rise of HbR and a depletion of HbO can be observed, which forms a major component of the OISI-signal (see previous chapter). Through the process of NVC, the initial regional depletion of oxygenated hemoglobin due to neuronal activation is met with a 1-2 second delayed hemodynamic response, which involves local capillary and adjoining arteriole dilatation and increase in CBV. This increase in CBV leads to a secondary increase in ‘fresh’ oxygenated hemoglobin as a form of overcompensation. In terms of spatial resolution, fMRI’s BOLD-dependency might have a relatively negative effect. While CBV-response often also involves surrounding arterioles, spatial resolution might be limited, as it might not necessarily reflect the ‘original’ site of activation. Alternatively, high-field fMRI does seem able to utilize only the initial dip in oxygenation, in parallel to OISI. As this is thought to be confined to the original site of neuronal activity, it allows functional imaging with a much higher spatial resolution than imaging based on delayed CBV-increases (6-8).
What is important to note here is that tumors can impair oxygenation and therefore the BOLD contrast, leading to additional biological grounds for reduction in spatial resolution or BOLD-sensitivity. Especially in the peri-tumoral tissue, vascular changes can lead to neurovascular uncoupling and therefore false negative responses, which are especially detrimental in the context of presurgical functional mapping (9).
Intra-operative applicability
Conventionally, fMRIs are acquired pre-operatively to determine potential functional consequences of tumor removal, including e.g. determining the hemispherical dominance for language (10). Important limitations of performing pre-operative fMRI-based mapping include the relatively low spatial resolution combined with the relatively limited types of functional tasks that can be performed in the scanner. Often, these remain limited to silent language-related tasks and small motor tasks, to prevent artifacts in the scanner.
In many institutions, fMRI is considered to be the gold standard for pre-operative functional mapping. However, the fMRI is rarely used in the intra-operative setting in combination with neuro-navigation to guide the neurosurgeon’s decision making. Intra-operatively, these images would have needed to be merged with the in-vivo brain anatomy in front of the surgeon. This is challenging as due to the inevitable brain shift after cranio- and durotomy, pre-operative images only provide a rough estimation of the 3D-locus of the tumor during surgery (11). Literature does, however, report successful use of fMRI-data intra-operatively in those cases where ESM was not possible or limited (12).
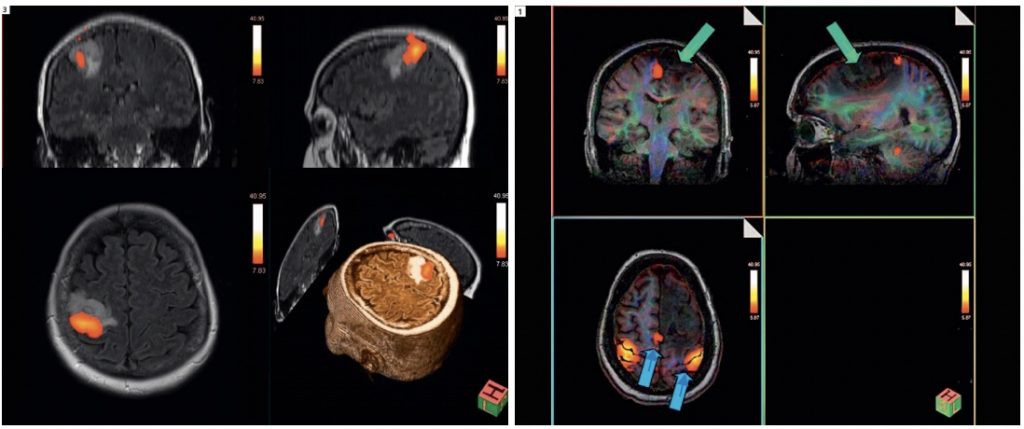
A limited number of institutions have access to so-called intra-operative MRI (iMRI) suites, where mostly structural MRI or DTI-scans are made during the surgical procedure to confirm the extent of resection (13). A very limited number of ifMRI or awake ifMRI (aifMRI) applications have been reported in literature (14), where during resection patients were subjected to functional tasks in the MRI-scanner. Although functional areas could be identified (Figure 3), the combination of susceptibility artefacts and severe disruption of surgical workflow, confirm that fMRI is a technique which serves its purpose in the surgical planning, but is not likely to be feasible intra-operatively.
References
- Ottenhausen M, Krieg SM, Meyer B, Ringel F. Functional preoperative and intraoperative mapping and monitoring: increasing safety and efficacy in glioma surgery. Neurosurg Focus. 2015;38(1):E3.
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008.
- Logothetis NK. The Underpinnings of the BOLD Functional Magnetic Resonance Imaging Signal. J Neurosci. 2018;
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annual Review of Physiology. 2004.
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion Tensor Imaging of the Brain. Neurotherapeutics. 2007;
- Zepeda A, Arias C, Sengpiel F. Optical imaging of intrinsic signals: Recent developments in the methodology and its applications. Journal of Neuroscience Methods. 2004.
- Kim DS, Duong TQ, Kim SG. High-resolution mapping of iso-orientation columns by fMRI. Nat Neurosci. 2000;
- Petridou N, Siero JCW. Laminar fMRI: What can the time domain tell us? Neuroimage. 2019;197.
- Pak RW, Hadjiabadi DH, Senarathna J, Agarwal S, Thakor N V., Pillai JJ, et al. Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors. Journal of Cerebral Blood Flow and Metabolism. 2017.
- Smits M, Visch-Brink E, Schraa-Tam CK, Koudstaal PJ, van der Lugt A. Functional MR imaging of language processing: an overview of easy-to-implement paradigms for patient care and clinical research. Radiographics. 2006;26 Suppl 1:S145-158.
- Soloukey S, Vincent AJPE, Satoer DD, Mastik F, Smits M, Dirven CMF, et al. Functional Ultrasound (fUS) During Awake Brain Surgery: The Clinical Potential of Intra-Operative Functional and Vascular Brain Mapping. Front Neurosci. 2020;
- Rigolo L, Essayed WI, Tie Y, Norton I, Mukundan S, Golby A. Intraoperative Use of Functional MRI for Surgical Decision Making after Limited or Infeasible Electrocortical Stimulation Mapping. J Neuroimaging. 2020;
- Ginat DT, Swearingen B, Curry W, Cahill D, Madsen J, Schaefer PW. 3 Tesla intraoperative MRI for brain tumor surgery. J Magn Reson Imaging.2014;39(6):1357–65.
- Lu JF, Zhang H, Wu JS, Yao CJ, Zhuang DX, Qiu TM, et al. “Awake” intraoperative functional MRI (ai-fMRI) for mapping the eloquent cortex: Is it possible in awake craniotomy? NeuroImage Clin.2013;2(1):132–42.
- Pillai JJ, Zaca D. Case Series: Presurgical Planning with fMRI/DT. Magnetom (Siemens). 2011;3.
- Duncan JS, Winston GP, Koepp MJ, Ourselin S. Brain imaging in the assessment for epilepsy surgery. The Lancet Neurology. 2016.
