fUS
Moderated by Sadaf Soloukey, PhD-student CUBE, department of Neuroscience and Neurosurgery, Erasmus MC, Rotterdam, the Netherlands.
Functional Ultrasound (fUS), also known as functional Ultrasound imaging (fUSi) is a relatively new technique which exploits a high-frame-rate (HFR) acquisition scheme to increase the sensitivity to detect subtle changes in motion, for example caused by moving blood cells. This increased sensitivity to motion now facilitates Power Doppler mapping of very small blood dynamics related to neuronal activity through the process of NVC (1–3).
Technical Parameters
Power Doppler imaging involves the previously discussed Doppler phenomenon and measures the backscattered ultrasonic energy from moving particles, such as RBCs, in each pixel of the image over time (1). fUS makes use of so-called HRF-imaging: through developments in hardware and software in experimental ultrasound acquisition units, thousands of successively tilted plane waves can be transmitted per second, which are coherently compounded into high-frame-rate images with much better spatiotemporal sensitivity. With this boost in power Doppler signal-to-noise-ratio, fUS is sensitive enough to detect blood dynamics in microvasculature down to 1 mm/s (1). Depending on the ultrasound frequency used, penetration depths of several centimeters are conventional (2,3). Several approaches to performing fUS in 3D or even 4D have also been reported in literature (4–6). Most approaches include volumetric fUS by acquiring and combining multiple scans using 2D-transducer arrays (4). The main challenges when moving to (real-time) volumetric data include maintaining high sensitivity and fast frame rates, while processing vast amounts of data.
Biological Substrate
The highly sensitive Power Doppler images (PDIs) acquired during fUS imaging allow for detection of miniscule blood dynamics in the brain’s venules, arterioles and especially capillaries. These blood dynamics include changes in blood flow (CBF), blood volume (CBV), as well as changes in vessel diameter due to relaxation and/or dilatation of the vessel wall. Through the principle of NVC, these changes in blood dynamics are thought to reflect changes in neuronal activity.
Intra-operative applicability
Given its portable, cheap and non-invasive nature combined with its unconventional micrometer-millisecond precision and high depth-penetration, make it a uniquely valuable technique for an intra-operative setting. So far, literature reports two successful intra-operative applications of fUS, for awake craniotomy functional mapping in particular (2,3) (see Figure 1 and 2). What is more, the resolution with which the brain’s microvascular morphology is captured makes fUS also a potential structural tool for tumor border delineation (2).
Outside of the OR, fUS is limited by the skull’s attenuation of sound waves. Nevertheless, transcranial Doppler ultrasound through thinner bone windows has proven to be a feasible method for monitoring CBF in the major intracranial arteries (7). Inside the operating room, the tissue directly under the craniotomy can be readily interrogated as long as good acoustic contact is maintained through using ultrasound gel and/or saline solution on the tissue of interest.
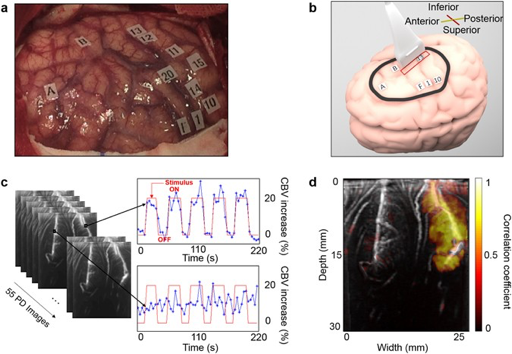
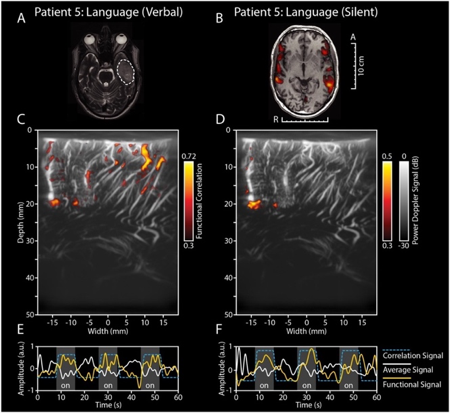
References
- Deffieux T, Demene C, Pernot M, Tanter M. Functional ultrasound neuroimaging: a review of the preclinical and clinical state of the art. Vol. 50, Current Opinion in Neurobiology. 2018. p. 128–35.
- Soloukey S, Vincent AJPE, Satoer DD, Mastik F, Smits M, Dirven CMF, et al. Functional Ultrasound (fUS) During Awake Brain Surgery: The Clinical Potential of Intra-Operative Functional and Vascular Brain Mapping. Front Neurosci. 2020;
- Imbault M, Chauvet D, Gennisson JL, Capelle L, Tanter M. Intraoperative Functional Ultrasound Imaging of Human Brain Activity. Sci Rep [Internet]. 2017;7(1):7304. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L626260218
- Brunner C, Grillet M, Sans-Dublanc A, Farrow K, Lambert T, Macé E, et al. A Platform for Brain-wide Volumetric Functional Ultrasound Imaging and Analysis of Circuit Dynamics in Awake Mice. Neuron. 2020;
- Rau R, Kruizinga P, Mastik F, Belau M, de Jong N, Bosch JG, et al. 3D functional ultrasound imaging of pigeons. Neuroimage. 2018;
- Rabut C, Correia M, Finel V, Pezet S, Pernot M, Deffieux T, et al. 4D functional ultrasound imaging of whole-brain activity in rodents. Nat Methods. 2019;
- Naqvi J, Yap KH, Ahmad G, Ghosh J. Transcranial Doppler ultrasound: A review of the physical principles and major applications in critical care. Vol. 2013, International Journal of Vascular Medicine. 2013.
