The following text serves as a temporary placeholder, prior to expert moderation
OISI
Moderated by: TBD
Optical Intrinsic Signal Imaging (OISI), also known as Intraoperative Optical Imaging (IOI) (1), is a scattering-based imaging technique which is dependent on several intrinsic signal changes of neural tissue during activation, including the oxygenation and deoxygenation of hemoglobin (1–4). Through these indirect measurements of neuronal activity, functional activation can be determined.
Technical Parameters
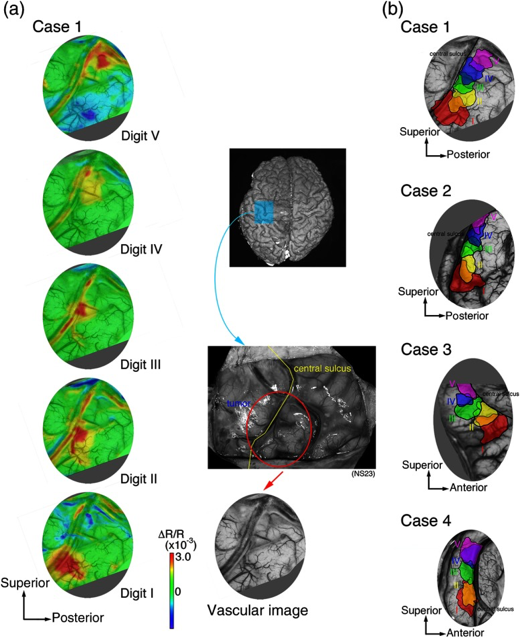
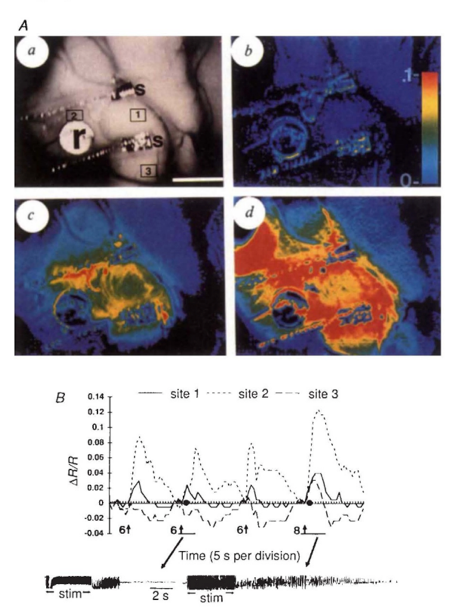
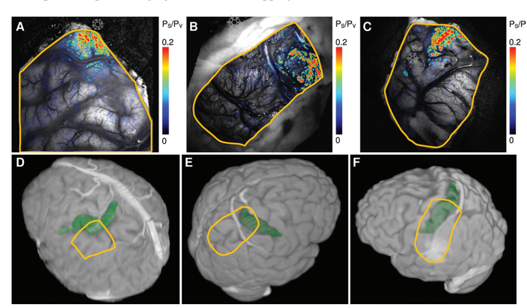
Like IFF-i, Ca-i, and VSD-i, OISI detects its intrinsic signals through a CCD-camera, the frame-rate of which mostly dictates the temporal resolution which can be achieved (milliseconds). In terms of spatial resolution, OISI performs relatively well, presenting with submillimeter precision. Like other optical techniques, penetrative depth is very limited, although it would allow to bring a large portion of the superficial cortex in the craniotomy into view at once (Figure 1-3). The signal itself is relatively small, and prone to artifacts such as motion which can quickly affect its signal to noise-ratio (1,3). Intra-operatively therefore, acquisition times in the range of minutes to gather enough data points seem conventional (1).
Biological Substrate
The source of the changes in intrinsic signal underlying OISI is thought to be at least threefold and consist of 1) changes in local CBV due to neuronal activation, 2) changes in oxygen saturation level of hemoglobin due to neuronal activation and 3) changes in light-scattering due to cortical activation (2,3). All three components serve as indirect measures of neuronal activity through the process of NVC, and are either metabolism- (1 and 2) or hemodynamics- (3) based. To interrogate the tissue for more hemodynamic- or metabolism-based signal, different wavelengths have to be used (1). For the changes in oxygen saturation levels, OISI makes use of the fact that oxygenated hemoglobin and deoxygenated hemoglobin have different absorption spectra, meaning that activated cortical areas will reflect light differently than non-activated areas (2). Through the process of NVC, the regional activation of neurons is met with an initial regional depletion of oxygenated hemoglobin or increase in de-oxygenated hemoglobin, which forms a major component of OISI.
Through the process of NVC, this original oxygen depletion is also met with a 1-2 second delayed hemodynamic response, which involves local and surrounding vessel dilatation and increase in CBV. This increase in CBV leads to a secondary increase in oxygenated hemoglobin as a form of overcompensation. This blood-oxygenation-level (BOLD)-signal is the basis other techniques such as fMRI, as will be discussed in the upcoming chapter. Thirdly, OISI depends on an increase in light scattering in activated areas as compared to non-activated ones, though to result from ion and water movement, capillary expansion or even neurotransmitter release (2). Together, these differences in intrinsic signal between activated and non-activated signal, facilitate a so-called mapping signal, which is often visualized as a fractional intensity change over time, converted into a color map which can be overlayed over the camera-image of the cortex (Figure 3).
Intra-operative applicability
Literature reports a number of in-human, intra-operative applications of OISI during oncological as well as epilepsy-related neurosurgical procedures (Figure 1-3) (1,3,5–12). Here, the main challenges revolve around limited penetration depths as well as motion artefacts, which make it difficult to detect the relatively small signal changes. For the latter, some authors suggest working with glass plates to stabilize the cortex, which in an intra-operative setting is far from ideal (1,3).
References
- Sobottka SB, Meyer T, Kirsch M, Koch E, Steinmeier R, Morgenstern U, et al. Intraoperative optical imaging of intrinsic signals: A reliable method for visualizing stimulated functional brain areas during surgery. J NEUROSURG.2013;119(4):853–63.
- Zepeda A, Arias C, Sengpiel F. Optical imaging of intrinsic signals: Recent developments in the methodology and its applications. Journal of Neuroscience Methods. 2004.
- Sato K, Nariai T, Momose-Sato Y, Kamino K. Intraoperative intrinsic optical imaging of human somatosensory cortex during neurosurgical operations. Neurophotonics. 2017;4(3).
- Lu HD, Chen G, Cai J, Roe AW. Intrinsic signal optical imaging of visual brain activity: Tracking of fast cortical dynamics. Neuroimage. 2017;
- Oelschlägel M, Meyer T, Morgenstern U, Wahl H, Gerber J, Reiß G, et al. Mapping of language and motor function during awake neurosurgery with intraoperative optical imaging. Neurosurg Focus. 2020;
- Prakash N, Uhlemann F, Sheth SA, Bookheimer S, Martin N, Toga AW. Current trends in intraoperative optical imaging for functional brain mapping and delineation of lesions of language cortex. Neuroimage. 2009;47(SUPPL. 2):T116–26.
- Cannestra AF, Bookheimer SY, Pouratian N, O’Farrell A, Sicotte N, Martin NA, et al. Temporal and topographical characterization of language cortices using intraoperative optical intrinsic signals. Neuroimage.2000;12(1):41–54.
- Nariai T, Sato K, Hirakawa K, Ohta Y, Tanaka Y, Ishiwata K, et al. Imaging of somatotopic representation of sensory cortex with intrinsic optical signals as guides for brain tumor surgery. J NEUROSURG.2005;103(3):414–23.
- Meyer T, Sobottka SB, Kirsch M, Schackert G, Steinmeier R, Koch E, et al. Intraoperative optical imaging of functional brain areas for improved image-guided surgery. Biomed Tech. 2013;58(3):225–36.
- Schwartz TH, Li MC, Friedman RM, Spencer DD, Roe AW. Intraoperative optical imaging of human face cortical topography: A case study. Neuroreport.2004;15(9):1527–31.
- Cannestra AF, Blood AJ, Black KL, Toga AW. The evolution of optical signals in human and rodent cortex. Neuroimage. 1996;
- Haglund MM, Ojemann GA, Hochman DW. Optical imaging of epileptiform and functional activity in human cerebral cortex. Nature. 1992;
