Ca-i
Moderated by Dr. Aleksandra Badura, Associate Professor Department of Neuroscience, Erasmus MC, Rotterdam, The Netherlands
Calcium-imaging (Ca-i) exploits the fact that depolarizing electrical signals in neurons depend on the influx of calcium through various voltage-gated Ca2+ channels (1,2). Calcium is a universal intracellular signal in eukaryotic cells, and the concentration of free calcium in cytoplasm is typically around 0.1𝜇M. This concentration is four orders of magnitude lower than the extracellular concentration and rises substantially after the action potential-driven calcium entry (Ca2+ signals can rise within 1 ms and fall in 10 to 100 ms in small subcellular structures) (3). By using Genetically Encoded Calcium Indicators (GECIs) or loading Ca2+ indicator dyes in the tissue of interest, the flux of calcium can be visualized using fluorescence as a measure of neuronal activity.
Technical Parameters
With Ca-i, we detect fluctuations in intracellular calcium expressed as fluorescence, as an indirect indicator of neuronal activity. To ensure the presence of these fluorescent calcium indicators in the tissue of interest, GECIs can be introduced in animal models using viral transduction techniques, in utero electroporation or by using genetically modified animal lines (1,2). In the dye-loading alternative, individual or multiple neurons are targeted by injecting the dye directly into the location of interest. Similar to VSD-i, the type of the indicator heavily influences the temporal resolution, signal-to-noise-ratio, and ultimately, the sensitivity of the imaging endeavor. Ca-i usually requires a fluorescence microscope, the camera of which mainly dictates the temporal resolution (millisecond), spatial resolution (<50 µm) and field of view that can be achieved (neuronal populations). Like most other optical imaging techniques, the penetration depth of Ca-i is limited (millimeter range).
Biological Substrate
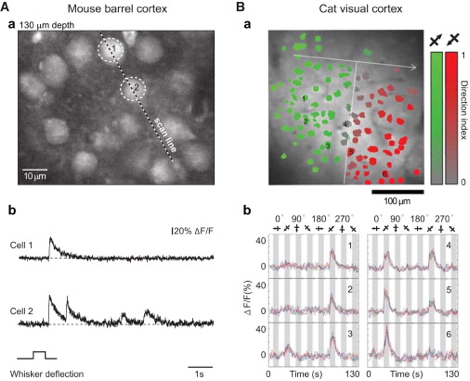
The influx of calcium is an essential component of several electrical events that underly neuronal activity, such as synaptic activation and dendritic spikes (4). By using calcium indicators in the cytoplasm, which become fluorescent when binding to calcium as it enters the cytoplasm, we have an indirect optical measurements of calcium concentration in the cytoplasm of the cell. This concentration is a direct indication of the presence of neuronal activity. One of the major biological challenges for Ca-i is the complex spatiotemporal dynamics of calcium transients, with different consequences of calcium influx in the soma, axon or dendrite. As usually only one of the compartments is visualized, a straightforward inference of the underlying electrical event is typically challenging (4). The conversion of calcium signals back to neural activity requires deconvolution algorithms and other statistical approaches to allow estimation of spike timing (3).
Intra-operative applicability
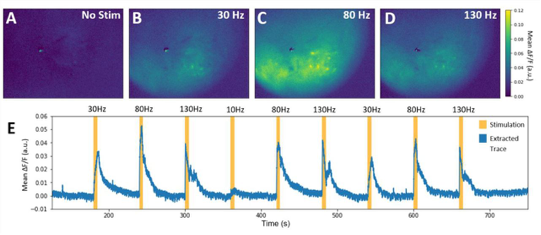
Similar to VSD-i, literature reports no intra-operative applications of Ca-i. Especially here, one of the most severe bottlenecks includes the need to introduce indicators, which in human applications will be difficult and invasive. Additionally, the very limited penetrative depth, as with most optical techniques, would only allow for very superficial detection of cortical functionality. In its neuroscientific application, however, Ca-i has proven successful, with applications in head-fixated as well as freely moving mice (5).
References
- Grienberger C, Konnerth A. Imaging Calcium in Neurons. Neuron. 2012.
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;
- Badura A, Sun XR, Giovannucci A, Lynch LA, Wang SS-H. Fast calcium sensor proteins for monitoring neural activity. Neurophotonics. 2014;1(2).
- Ali F, Kwan AC. Interpreting in vivo calcium signals from neuronal cell bodies, axons, and dendrites: a review. 2019;
- Trevathan JK, Asp AJ, Nicolai EN, Trevathan JM, Kremer NA, Kozai TD, et al. Calcium imaging in freely-moving mice during electrical stimulation of deep brain structures. bioRxiv.
