IFF-i
Moderated by Dr. Joost Jongen, researcher and neurologist, Erasmus MC, Rotterdam, The Netherlands
Intrinsic Functional Fluorescence imaging (IFF-i) is a technique which exploits the fact that metabolic molecules such as Nicotinamide Adenine Dinucleotide (NADH), Flavin Adenine Dinucleotide (FAD) or Flavoprotein present with endogenous fluorescence. Imaging the presence of metabolism-related changes in fluorescence can be used as an indirect measure of local neuronal activity, through the process of NVC (1).
Technical Parameters
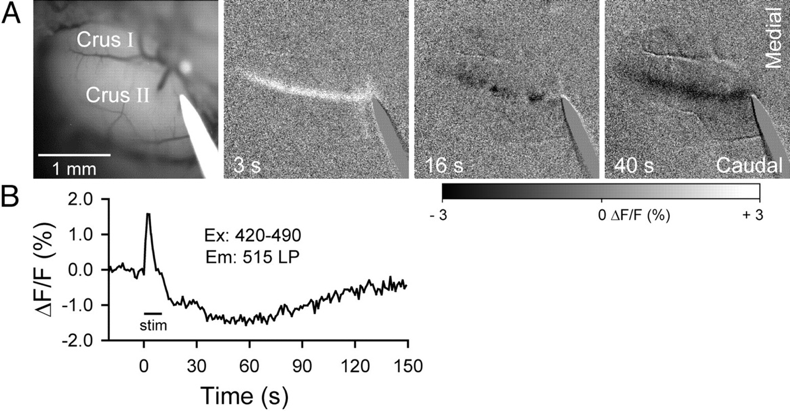
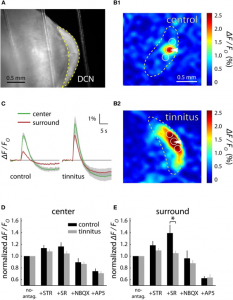
In all three endogenous fluorophore-based techniques, the signal is detected by a Charge Coupled Device (CCD)-camera, amplified, processed and often displayed as a fractional intensity change over time, similar to VSD (Figure 1 and 2). Again, the technique’s temporal resolution is dependent on the camera (millisecond). Its spatial resolution, like most optical techniques, is consideredable<500 µm). However, the penetrative depth is again limited to a millimeter.
Biological Substrate
There are a limited number of endogenous fluorophores present in neurons, of which metabolic reducing proteins form an important part (2). Autofluorescent Flavoprotein imaging (AFi) relies on the intrinsic fluorescent signal of oxygenated mitochondrial flavoprotein (1,3,4).
In AFI, this oxidization serves as an indirect measure of the aerobic energy metabolism following neuronal activation. In fact, there is a linear relationship between the fluorescence signals and neuronal activity (5). Generally the signal intensity of AFi is considered to be higher as compared to NADH or Optical Intrinsic Signal Imaging (OISI) (see next chapter) (6). NADH is a molecule involved in the glucose metabolism and is created when two NAD+ molecules are reduced to NADH in order to convert glucose to pyruvate in the cytoplasm during glycolysis (1,4). Detecting the presence of fluorescent NADH over time can therefore serve as a measure of glucose metabolism rates, and indirectly through the process of NVC, serve as a measure of neuronal activity.
Intra-operative applicability
Similar to SVD-i and Ca-i , literature reports no intra-operative applications of IFF-i. In animal preparations, AFi has been mostly applied to ex vivo brain slices (Figure 1) or anesthetized animals, with reports on its limitations such as its penetrative depth, combined with its susceptibility to motion artifacts (6). Therefore, for intra-operative neurosurgical use, IFF-i does not seem particularly suitable.
References
- Husson TR, Issa NP. Functional Imaging with Mitochondrial Flavoprotein Autofluorescence: Theory, Practice, and Applications. In Vivo Optical Imaging of Brain Function. 2009.
- Reinert KC, Dunbar RL, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging of neuronal activation in the cerebellar cortex in vivo. J Neurophysiol. 2004;
- Michael N, Bischof HJ, Löwel S. Flavoprotein autofluorescence imaging of visual system activity in zebra finches and mice. PLoS One. 2014;
- Kolenc OI, Quinn KP. Evaluating cell metabolism through autofluorescence imaging of NAD(P)H and FAD. Antioxidants and Redox Signaling. 2019.
- Shibuki K, Hishida R, Murakami H, Kudoh M, Kawaguchi T, Watanabe M, et al. Dynamic imaging of somatosensory cortical activity in the rat visualized by flavoprotein autofluorescence. Journal of Physiology. 2003.
- Jongen JLM, Pederzani T, Koekkoek SK, Shapiro J, Van Der Burg J, De Zeeuw CI, et al. Autofluorescent flavoprotein imaging of spinal nociceptive activity. J Neurosci. 2010;
- Middleton JW, Tzounopoulos T. Imaging the neural correlates of tinnitus: A comparison between animal models and human studies. Front Syst Neurosci. 2012;
