The following text serves as a temporary placeholder, prior to expert moderation
LSCI
Moderated by: TBD
Laser Speckle Contrast Imaging (LSCI) is a scattering-based optical technique which makes use of the fact that laser illumination of tissue creates a random speckle pattern (1). Movement of scatterers, such as red blood cells, can lead to observable fluctuations in this speckle pattern, and as such, LSCI allows for measurements of CBF (1,2).
Technical Parameters
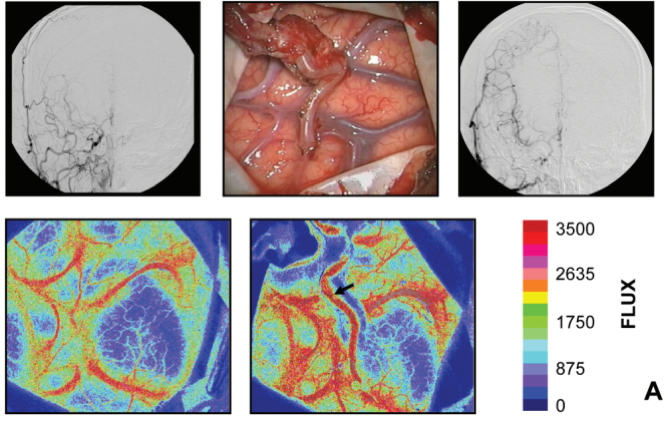
The speckle pattern which forms the basis of LSCI is the consequence of random interference of coherent light (3,4). When a coherent light source is sent on tissue, its photons will travel through that tissue with slightly different path lengths (3,4), which can lead to varying interference in space. This random interference will produce a randomly varying intensity pattern known as speckle, which can be detected by the photodetector of a camera (3,4). Again, when the light scatters of moving particles such as RBCs, this will cause temporal fluctuations in the interference, and therefore lead to intensity or contrast variations in the speckle pattern, from which we can infer the speeds of the blood flow responsible for these fluctuations (3,4). Similar to LDF, LSCI is restricted by the penetration depth and backscattering of light and is therefore limited to superficial vessels only, with penetration depths of not much more than one millimeter (5). The spatial resolution, however can go down to <50 µm (5) and is dependent on both the pixel size of the camera used to detect the pattern, as well as the minimum speckle size, which is directly proportional to the laser wavelength (4). Often, the contrast images are processed and presented as color-coded live-images reflecting the different blood flow velocities present in the tissue (6) (Figure 1 and 2).
LSCI like other optical techniques is restricted by the penetration depth of light and is therefore limited to superficial vessels only, with penetration depths of not much more than one millimeter (5). The spatiotemporal resolution of the technique can be tailored to specific applications. The spatial resolution of LSCI can go down to <50 µm. In the temporal domain, the resolution is dictated by the frame rate of the imaging device (such as the camera), ranging from milliseconds to several seconds (5). LSCI is able to cover the full superficial cortical surface revealed by the craniotomy (e.g. full brain lobe), limited only by the aperture of the camera in combination with the field which can be covered by the laser. Intra-operative acquisition time sufficient to find functional correlations seems to be in the range of minutes per single functionality or functional task (7).
Biological Substrate
When photons are scattered by moving particles such as red blood cells in cortical vessels, they create detectable fluctuations in the speckle pattern, which in turn can be used to infer the speeds at which these particles were moving. In other words, LSCI can be used as a measure for CBF in superficial cortical vessels and brain perfusion in general. LSCI is reported to be mostly sensitive to CBF-changes in brain microvasculature, such as capillaries (3,6,8,9).
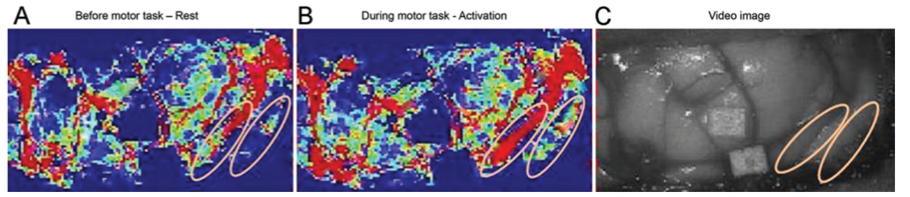
Similar to other NVC-based techniques such as fNIRS and fMRI, combining CBF-measurements using LSCI with functional tasks of a known pattern, would allow for identification of functional regions in an intra-operative setting (7). The fact that LSCI is a video-based technique, enables parallel acquisition of images of vascular morphology. This facilitates the opportunity to e.g. superimpose functional maps over structural images (Figure 1 and 2).
Intra-operative applicability
LSCI is a particularly cheap, safe, portable and non-invasive option, which provides both high-resolution functional as well as vascular morphological information in an intra-operative setting. LSCI has been tested in an experimental intra-operative setting on multiple occasions for neurovascular as well awake neuro-oncological procedures using commercial and experimental devices often consisting of compact laser-camera combinations (3,6,7). Currently, the literature describes trends toward development of more integrated devices, such as displaying structural-functional LSCI-based images directly onto the eyepiece of a surgical microscope in combination with real-time video-angiography (8). Although these developments seem promising, LSCI is inherently bottlenecked by its limited depth penetration, and can therefore only provide superficial cortical information. Additionally, literature reports the conventionally known challenge of correcting for pulsatile motion artifacts due to the patient’s heartbeat and breathing (10).
References
- Briers D, Duncan DD, Hirst E, Kirkpatrick SJ, Larsson M, Steenbergen W, et al. Laser speckle contrast imaging: theoretical and practical limitations. J Biomed Opt. 2013;
- Stern MD. In vivo evaluation of microcirculation by coherent light scattering. Nature. 1975;
- Parthasarathy AB, Weber EL, Richards LM, Fox DJ, Dunn AK. Laser speckle contrast imaging of cerebral blood flow in humans during neurosurgery: a pilot clinical study. J Biomed Opt. 2010;15(6):66030.
- Boas DA, Dunn AK. Laser speckle contrast imaging in biomedical optics. J Biomed Opt. 2010;
- Hecht N, Woitzik J, Dreier JP, Vajkoczy P. Intraoperative monitoring of cerebral blood flow by laser speckle contrast analysis. Neurosurg Focus. 2009;27(4):E11.
- Senarathna J, Rege A, Li N, Thakor N V. Laser speckle contrast imaging: Theory, instrumentation and applications. IEEE Rev Biomed Eng. 2013;
- Klijn E, Hulscher HC, Balvers RK, Holland WPJ, Bakker J, Vincent AJPE, et al. Laser speckle imaging identification of increases in cortical microcirculatory blood flow induced by motor activity during awake craniotomy ; Clinical article. J NEUROSURG. 2013;118(2):280–6.
- Mangraviti A, Volpin F, Cha J, Cunningham SI, Raje K, Brooke MJ, et al. Intraoperative Laser Speckle Contrast Imaging For Real-Time Visualization of Cerebral Blood Flow in Cerebrovascular Surgery: Results From Pre-Clinical Studies. Sci Rep. 2020;
- Klijn E, Hulscher HC, Balvers RK, Holland WP, Bakker J, Vincent AJ, et al. Laser speckle imaging identification of increases in cortical microcirculatory blood flow induced by motor activity during awake craniotomy. J Neurosurg.2013;118(2):280–6.
- Parthasarathy AB, Weber EL, Richards LM, Fox DJ, Dunn AK. Laser speckle contrast imaging of cerebral blood flow in humans during neurosurgery: a pilot clinical study. J Biomed Opt. 2010;15(6):66030.
