OCT
Moderated by Dr. Mitra Almasian, postdocotoral researcher Amsterdam UMC, The Netherlands
Optical Coherence Tomography (OCT) is a scattering-based optical technique. (1–3). The contrast in OCT images is determined by the refractive index differences in tissue such as the brain. Combined with the Doppler principle, a functional extension of OCT called Doppler OCT (D-OCT) allows for volumetric reconstruction of high-resolution tomographic images of cerebral blood flow (CBF) in response to neuronal activation (2).
Technical Parameters
OCT is an imaging technique comparable to ultrasound imaging but using light waves instead of sound waves (3). It stools on the principle of interference: a light beam from a source is split in two, where one beam is considered the ‘reference’ beam and the other the ‘sample’ beam focused on the sample. Dependent on the refractive index of the tissue and size of scattering particles, a fraction of the light is back scattered. The back scattered light is detected and recombined with the “reference” beam, which produces an interference pattern (4). The interference pattern contains depth information, which allows for a morphological reconstruction of the depth of a sampled tissue section (A-scan). Multiple neighboring A-scans combine to form a 2D B-scan, which can be seen as an analogue to the B-mode in ultrasound. Volumetric OCT is usually obtained by combining multiple B-scans in a 3D volume (5). When combining this structural information with neurophysiological information gained from the Doppler signal (see ‘LDF’), the functional alternative ‘D-OCT’ is created. D-OCT has the ability to detect quantitative measures of CBF in addition to the morphological tissue information (see Figure 1).
In terms of spatial resolution, OCT uses broad width white light sources, allowing for micrometer precision (4). Its penetrative depth, however, is limited to 1-2 millimeters, as at greater depths the proportion of light that is scattered is not detectable. Depending on the wavelength and the sampled tissue, absorption can also play a role in the decrease of OCT signal with depth. The technique’s temporal resolution depends on the scanning speed of a single 2D B-scan and scanning volume that needs to be covered but can be as low as milliseconds.
Biological Substrate
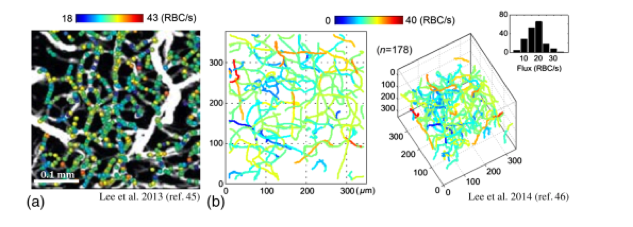
The unique characteristic of D-OCT is its potential to combine high-resolution structural imaging with quantitative volumetric measures of CBF. Similar to LDF, these CBF measurements serve as an indirect indication of neuronal activation, allowing functional measurements with D-OCT. On top of this, the structural OCT scan allows for morphological information of vascular structures with micrometer precision. Segmentation of blood vessels from OCT-datasets is therefore also known as ‘OCT Angiography’ or OCTA (6).
Intra-operative applicability
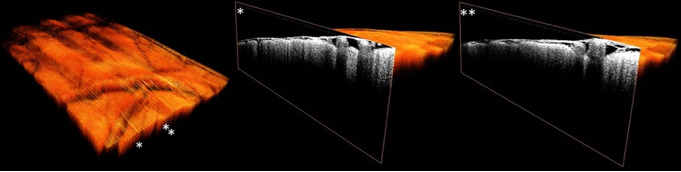
D-OCT is a non-invasive technique which is able to combine volumetric structural and functional information in real-time with high temporal and spatial resolution. With this particular set of characteristics, it would fit well in the intra-operative context, already proven by the fact that OCT is used regularly surgical ophthalmological procedures using portable, hand-held OCT-probes or systems integrated into conventional surgical microscopes (7,8). What is less ideal in the neurosurgical context, however, is the technique’s penetrative depth combined with its field of view, which will only allow detection of discrete regions the superficial cortex at best. For cerebral applications, OCT so far shows more potential as a structural technique to distinguish brain form pathological tissue such as tumor (see Figure 2) (9,10). Additionally, endovascular-OCT for in vivo volumetric microscopy in cerebrovascular procedures, seems promising (11).
References
- Srinivasan VJ, Sakadžić S, Gorczynska I, Ruvinskaya S, Wu W, Fujimoto JG, et al. Depth-resolved microscopy of cortical hemodynamics with optical coherence tomography. Opt Lett. 2009;
- Gangjun Liu GL, and Zhongping Chen and ZC. Advances in Doppler OCT. Chinese Opt Lett. 2013;
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical Coherence Tomography. Science (80- ). 1991;254:1178–81. A
- Wachulak P, Bartnik A, Fiedorowicz H. Optical coherence tomography (OCT) with 2 nm axial resolution using a compact laser plasma soft X-ray source. Sci Rep. 2018;
- Gabriele ML, Wollstein G, Ishikawa H, Xu J, Kim J, Kagemann L, et al. Three dimensional optical coherence tomography imaging: Advantages and advances. Progress in Retinal and Eye Research. 2010.
- Leitgeb RA, Werkmeister RM, Blatter C, Schmetterer L. Doppler Optical Coherence Tomography. Progress in Retinal and Eye Research. 2014.
- Gregori NZ, Lam BL, Davis JL. Intraoperative Use of Microscope-Integrated Optical Coherence Tomography for Subretinal Gene Therapy Delivery. Retina. 2019;
- Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;
- Kut C, Chaichana KL, Xi J, Raza SM, Ye X, McVeigh ER, et al. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci Transl Med. 2015;7(292).
- Almasian M, Wilk LS, Bloemen PR, van Leeuwen TG, ter Laan M, Aalders MCG. Pilot feasibility study of in vivo intraoperative quantitative optical coherence tomography of human brain tissue during glioma resection. J Biophotonics. 2019;
- Ughi GJ, Marosfoi MG, King RM, Caroff J, Peterson LM, Duncan BH, et al. A neurovascular high-frequency optical coherence tomography system enables in situ cerebrovascular volumetric microscopy. Nat Commun. 2020;
- Baran U, Wang RK. Review of optical coherence tomography based angiography in neuroscience. Neurophotonics. 2016;
